Hypothetical biochemical types are biochemical forms that speculate to be scientifically viable but not proven at this time. The types of living organisms currently known on Earth all use carbon compounds for basic structural and metabolic functions, water as solvents, and DNA or RNA to define and control their shape. If life exists on another planet or moon, it may be chemically similar; There is also the possibility that there are organisms with very different chemicals - for example, involving other classes of carbon compounds, other elemental compounds, or other solvents in aquatic place.
The possibility of life forms based on "alternative" biochemistry is a topic of ongoing scientific discussion, informed by what is known about the space environment and about the chemical behavior of various elements and compounds. It is also a common subject in science fiction.
The element of silicon has been widely discussed as a hypothetical alternative to carbon. Silicon is in the same group as carbon in the periodic table and, like carbon, it is tetravalent, although the silicon analogs of organic compounds are generally less stable. Hypothetical alternatives to water include ammonia, which, like water, is a polar molecule, and is cosmically abundant; and non-polar hydrocarbon solvents such as methane and ethane, which are known to exist in liquid form on the Titan surface.
Video Hypothetical types of biochemistry
Shadow biosphere
Shadow biosphere is a hypothetical microbial biosphere of Earth that uses a very different biochemical and molecular process from life known today. Although life on Earth is relatively well studied, the shadow biosphere may still remain unknown because the exploration of the microbial world primarily targets the biochemistry of macro-organisms.
Maps Hypothetical types of biochemistry
Alternative biomolecules-chirality
Perhaps the most unusual alternative biochemistry will be one with a different chirality from its biomolecules. In a known Earth-based life, the amino acids are almost universally of the form L and sugar is the form D . The opposite chirality molecules have chemical properties identical to their mirror shape, so life using D amino acids or L sugars is possible; such chirality molecules, however, would be incompatible with organisms using opposite chirality molecules. Amino acids that are chirality contrary to norms are found on Earth, and these substances are generally thought to result from the decay of normal chiral organisms. However, physicist Paul Davies speculates that some of them may be products of "anti-chiral" life.
However, it is questionable whether such biochemicals will be completely alien. Though of course it will be alternative stereochemistry, the molecules highly found in one enantiomer in most organisms can still be found in other enantiomers in different (often basal) organisms as in comparison between Archaea members and other domains, making it an open topic whether a stereochemistry a completely new alternative.
Non-carbon biochemistry
On Earth, all known living things have carbon-based structures and systems. Scientists have speculated about the pros and cons of using atoms other than carbon to form the molecular structures necessary for life, but nobody proposes a theory that uses these atoms to form all the necessary structures. However, as Carl Sagan says, it is very difficult to ascertain whether the statements that apply to all life on Earth will apply to all life throughout the universe. Sagan uses the term "carbon chauvinism" for such assumptions. He considers silicon and germanium as possible alternatives to carbon; but, on the other hand, he notes that carbon appears to be more chemical and more abundant in the universe.
Silicon biochemistry
Silicon atoms have been widely discussed as the basis for alternative biochemical systems, because silicon has many chemical properties that are similar to carbon and are in the same group of periodic tables, carbon groups. Like carbon, silicon can create molecules large enough to carry biological information.
However, silicon has several disadvantages as an alternative to carbon. Silicon, unlike carbon, lacks the ability to form chemical bonds with different types of atoms as necessary for the chemical flexibility necessary for metabolism. The elements that make up the organic functional group with carbon include hydrogen, oxygen, nitrogen, phosphorus, sulfur, and metals such as iron, magnesium, and zinc. Silicon, on the other hand, interacts with very few other types of atoms. In addition, where it interacts with other atoms, silicon creates molecules that have been described as "monotonous compared to the organic macromolecular combinatorial universe". This is because the silicon atom is much larger, has a larger mass and atomic radius, so it has difficulty forming a double bond (carbon double bond is part of the carbonyl group, a fundamental motif of bio-organic chemistry).
Silan, which is a chemical compound of hydrogen and silicon analogous to alkane hydrocarbons, is highly reactive with water, and long-chain silane spontaneously decomposes. Molecules that combine polymers of silicon and oxygen atoms alternately in lieu of the direct bonds between silicon, known collectively as silicon, are much more stable. It has been suggested that silicon-based chemicals will be more stable than equivalent hydrocarbons in sulfur-rich environments, such as those found in some space locations.
Of the various types of molecules identified in interstellar mediums in 1998, 84 are based on carbon while only 8 are based on silicon. In addition, of the 8 compounds, four also include carbon in it. The cosmic abundance of carbon to silicon is about 10 to 1. This may suggest more variation of complex carbon compounds throughout the cosmos, providing fewer foundations for building silicon-based biology, at least under the prevalent conditions on the surface. of the planet. Also, although Earth and other terrestrial planets are very rich in silicon and carbon (the relative abundance of silicon to carbon in the Earth's crust is approximately 925: 1), terrestrial life is carbon-based. The fact that carbon is used instead of silicon, may be evidence that silicon is unsuitable for biochemistry on Earth-like planets. The possible reason that silicon is less versatile than carbon in forming compounds, is that the compound formed by silicon is unstable, and it inhibits heat flow.
Even so, biogenic silica is used by some Earth life, such as the structure of the diatom silicate skeleton. According to the Cairns-Smith A.G clay hypothesis, silicate minerals in water play an important role in abiogenesis: they replicate their crystal structure, interact with carbon compounds, and are carbon-based life precursors.
Although not observed in nature, carbon-silicon bonds have been added to biochemistry by using directional evolution (artificial selection). A heme containing cytochrome protein c from Rhodothermus marinus has been engineered using directional evolution to catalyze the formation of new carbon-silicon bonds between the hydrosilan and diazo compounds.
Silicon compounds may be biologically useful under different temperatures or pressures from the surface of terrestrial planets, either in relation to or in a role that is less directly analogous to carbon. Polysilanol, a sugar-related silicone compound, dissolves in liquid nitrogen, shows that they can play a role in very low temperature biochemistry.
In cinematic and literary science fiction, as human-made machines move from non-life to life, it is often argued that this new form will be the first example of non-carbon-based life. Since the advent of microprocessors in the late 1960s, these machines have often been classified as computers (or computer-guided robots) and stored under "silicon-based life", although the silicon-support matrix of these processors is not nearly as fundamental. Their operation as carbon is for "wet life".
Biochemistry of other exotic elements
- Borans are dangerous explosives in Earth's atmosphere, but will be more stable in a reducing environment. However, the low cosmic abundance of boron makes it less likely to be the basis of life than carbon.
- A variety of metals, together with oxygen, can form highly complex and thermally stable structures that rival organic compounds; Heteroid acid is one such family. Some metal oxides are also similar to carbon in their ability to form nanotube and crystalline structures such as diamonds (such as cubic zirconia). Titanium, aluminum, magnesium, and iron are more abundant in the earth's crust than carbon. Therefore, life-based metal oxides can be a possibility under certain conditions, including those (such as high temperatures) where carbon-based life would not be possible. The Cronin group at the University of Glasgow reported self-assembly tungsten polyoxometalates into cells such as spheres. By modifying their metal oxide content, the sphere can obtain a hole that acts as a porous membrane, selectively allowing chemicals to enter and exit the ball according to size.
- Sulfur is also able to form long chain molecules, but also suffers from high reactivity issues such as phosphorus and silane. The biological use of sulfur as an alternative to carbon is purely hypothetical, especially since sulfur usually forms only linear chains rather than branched ones. (The biological use of sulfur as an electron acceptor is widespread and can be traced back 3.5 billion years on Earth, thus preceding the use of molecular oxygen.Sulfur-reducing bacteria can use sulfur instead of oxygen, reducing sulfur to hydrogen sulfide.)
Arsenic as an alternative to phosphorus
Arsenic, which is chemically similar to phosphorus, is temporarily toxic to most life forms on Earth, incorporated into the biochemistry of several organisms. Some seaweeds enter arsenic into complex organic molecules such as arsenosugars and arsenobetaines. Fungi and bacteria can produce volatile alcohol arsenic compounds. Arsenic reduction and arsenite oxidation have been observed in microbes ( Chrysiogenes arsenatis ). In addition, some prokaryotes may use arsenic as a terminal electron acceptor during anaerobic growth and some may utilize arsenite as an electron donor to generate energy.
It has been speculated that the earliest life forms on Earth may have used arsenic in the phosphorus place in their DNA structure. A common objection to this scenario is that the ester arsenate is much less stable for hydrolysis than the corresponding phosphate ester which arsenic is less suited for this function.
The authors of the 2010 study of geomycrobiology, supported in part by NASA, have postulated that a bacterium, named GFAJ-1, collected in Lake Mono sediments in eastern California, can use such 'arsenic DNA' when cultured without phosphorus. They propose that bacteria can use high levels of poly -? - hydroxybutyrate or other means of reducing the effective concentration of water and stabilizing the arsenic ester. This claim was strongly criticized immediately after publication due to lack of appropriate controls. Science writer Carl Zimmer contacted several scientists for the assessment: "I contacted a dozen experts... Almost unanimously, they think NASA scientists have failed in making their case". Other authors can not reproduce their results and show that studies have problems with phosphate contamination, suggesting that these low numbers can sustain extremophile life forms. Alternatively, it is recommended that GFAJ-1 cells grow by recycling the phosphate of the degraded ribosome, rather than by replacing it with arsenate.
Non-aqueous solution
In addition to carbon compounds, all known terrestrial life today also requires water as a solvent. This has led to a discussion of whether water is the only fluid capable of filling that role. The idea that extraterrestrial life might be based on solvents other than water has been taken seriously in recent scientific literature by biochemist Steven Benner, and by an astrobiological committee headed by John A. Baross. The solvents discussed by the Baross committee include ammonia, sulfuric acid, formamide, hydrocarbons, and (at a much lower temperature than Earth) liquid nitrogen, or hydrogen in the form of supercritical fluids.
Carl Sagan once described himself as a carbon chauvinist and a water chauvinist; However on another occasion he said he was a carbon chauvinist but "not much water chauvinist". He speculated about hydrocarbons, hydrofluoric acid, and ammonia as a possible alternative to water.
Some important water properties for life processes include the large temperature range in which the liquid, high heat capacity (useful for temperature regulation), large heat of evaporation, and the ability to dissolve various compounds. Water is also amphoteric, meaning it can donate and receive H ions, allowing it to act as acid or base. This property is very important in many organic and biochemical reactions, where water serves as a solvent, reactant, or product. There are other chemicals of a similar nature that are sometimes proposed as alternatives. In addition, water has unusual properties that are less dense as solids (ice) than as liquids. This is why the water body freezes but does not freeze solid (from bottom to top). If the ice is denser than liquid water (as is true for almost any other compound), then the large body of the liquid will slowly solidify, which will not be conducive to the formation of life. Water as a compound is cosmically abundant, though mostly in the form of steam or ice. The subsurface fluids are thought to be possible or possibly on several outer months: Enceladus (where geysers have been observed), Europa, Titan, and Ganymede. Earth and Titan are the only worlds currently known to have a stable liquid body on its surface.
However, not all water properties are beneficial to life. For example, water ice has a high albedo, meaning that it reflects a large amount of light and heat from the Sun. During the ice age, since reflective ice accumulated above the water surface, the effects of global cooling increased.
There are several properties that make certain compounds and elements far more profitable than others as solvents in a successful biosphere. Solvents must be able to exist in liquid equilibrium at various temperatures to be encountered by planetary objects. Since the boiling point varies with pressure, the question tends not to do the prospective solvent remains liquid, but under what pressure. For example, hydrogen cyanide has a narrow liquid phase temperature range at 1 atmosphere, but in an atmosphere with Venus pressure, with 92 bar (91Ã, atm) pressure, it can indeed exist in liquid form over a wide temperature range.
Ammonia
The ammonia molecule (NH 3 ), like water molecules, is abundant in the universe, being the hydrogen compound (the simplest and most common element) with another very common element, nitrogen. The possible role of liquid ammonia as an alternative solvent for life is an idea that goes back at least to 1954, when J.B.S. Haldane raised the topic on the symposium about the origin of life.
Many chemical reactions are possible in ammonia solution, and liquid ammonia has a chemical similarity to water. Ammonia can dissolve most of the organic molecules at least as well as water and, in addition, it is capable of dissolving many metallic elements. Haldane made the point that various organic compounds related to common water have analogues associated with ammonia; eg amine groups associated with ammonia (-NH 2 ) analogous to hydroxyl groups associated with water (-OH).
Ammonia, like water, can receive or donate H ions. When ammonia receives H , it forms an ammonium cation (NH 4 ), analogous to hydronium (H 3 O < soup> ). When he donates the H ion, he forms an amide anion (NH 2 - ), analogous to the hydroxide anion (OH - ). However, compared to water, ammonia is more likely to receive H ions, and less likely to contribute one; it is a stronger nucleophile. Ammonia is added to the water function as Arrhenius base: increasing the concentration of anion hydroxide. Instead, using the definition of solvent and alkaline solvent systems, water is added to the function of liquid ammonia as an acid, since it increases the concentration of ammonium cations. The carbonyl group (C = O), widely used in terrestrial biochemistry, will not be stable in the ammonia solution, but the analog imino group (C = NH) may be used instead.
However, ammonia has some problems as the basis of life. The hydrogen bond between ammonia molecules is weaker than water, causing the heat of ammonia evaporation to be half the water, its surface tension to one-third, and reducing its ability to concentrate non-polar molecules through hydrophobic effects. Gerald Feinberg and Robert Shapiro have questioned whether ammonia can bring prebiotic molecules together well enough to allow the emergence of self-reproduction systems. Ammonia is also flammable in oxygen, and can not exist sustainably in an environment suitable for aerobic metabolism.
An ammonia-based biosphere is likely to be present at very unusual temperatures or air pressure in relation to life on Earth. Life on Earth is usually present in the melting point and boiling point of water at normal pressure, between 0 ° C (273 ° K) and 100 ° C (373 ° K); at the melting point and boiling point the normal ammonia pressure is between -78 ° C (195 ° K) and -33 ° C (240 ° K). Chemical reactions generally take place more slowly at lower temperatures. Therefore, ammonia-based life, if anything, may metabolize more slowly and evolve more slowly than life on Earth. On the other hand, lower temperatures can also allow living systems to use chemical species that would be too labile at Earth's temperatures to be useful.
Ammonia can become liquid at temperatures like the Earth, but at much higher pressures; for example, at 60 atm, the ammonia melts at -77 ° C (196 ° K) and boils at 98 ° C (371 ° K).
The ammonia and ammonia-water mixture remains liquid at temperatures well below the freezing point of pure water, so such biochemistry may be suitable for planets and moons orbiting outside the water-based feasibility zone. Such conditions could exist, for example, beneath the surface of Titan's largest moon of Saturn.
Methane and other hydrocarbons
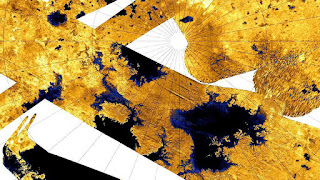
Methane (CH 4 ) is a simple hydrocarbon: a compound of the two most common elements in the cosmos, hydrogen and carbon. It has a cosmic abundance comparable to ammonia. Hydrocarbons can act as solvents at various temperatures, but will lack polarity. Isaac Asimov, a biochemist and science fiction writer, suggested in 1981 that poly-lipids can form protein substitutes in non-polar solvents such as methane. Lakes composed of a mixture of hydrocarbons, including methane and ethane, have been detected on the surface of Titan by Cassini spacecraft .
There is a debate about the effectiveness of methane and other hydrocarbons as a solvent for life compared to water or ammonia. Water is a stronger solvent than hydrocarbons, allowing the transport of substances more easily in cells. However, water is also more chemically reactive, and can break down large organic molecules through hydrolysis. The solvent life form is that hydrocarbons will not face the threat of biomolecules being destroyed in this way. Also, the tendency of water molecules to form strong hydrogen bonds can disrupt internal hydrogen bonds in complex organic molecules. Living with a hydrocarbon solvent can make more use of hydrogen bonding in its biomolecules. In addition, the strength of the hydrogen bond in the biomolecule will correspond to the biochemistry of low temperatures.
Astrobiologist Chris McKay argues, on the basis of thermodynamics, that if life existed on the surface of Titan, using hydrocarbons as solvents, it is also possible to use more complex hydrocarbons as energy sources by reacting them with hydrogen, reducing ethane and acetylene to methane. The possible evidence for this life form on Titan was identified in 2010 by Darrell Strobel of Johns Hopkins University; the greater molecular hydrogen abundance in Titan's upper atmosphere layer compared with the bottom layer, arguing for downward diffusion at a rate of about 10 molecules per second and the loss of hydrogen near the Titan surface. As Strobel noted, his findings are in line with the effect that Chris McKay predicted if methanogenic life forms were present. That same year, another study showed low levels of acetylene on the Titan surface, interpreted by Chris McKay consistent with the hypothesis of organisms that reduce acetylene to methane. Reaffirming biological hypotheses, McKay cautions that other explanations for the findings of hydrogen and acetylene should be considered more likely: the possibility of unidentified physical or chemical processes (eg non-living surface catalysts that allow acetylene to react with hydrogen), or deficiencies in current flow models material. He notes that even an effective non-biological catalyst at 95 K will itself be a surprising discovery.
Azotosome
A hypothetical cell membrane called azotosome capable of functioning in liquid methane under Titan conditions is a computer-model in a paper published in February 2015. Consisting of acrylonitrile, a small molecule containing carbon, hydrogen, and nitrogen, predicted to have stability and flexibility in liquid methane in proportion to the phospholipid bilayer (the type of cell membrane held by all life on Earth) in liquid water. Analysis of data obtained using Atacama Large Millimeter/submillimeter Array (ALMA), completed in 2017, confirmed a large amount of acrylonitrile in Titan's atmosphere.
Hydrogen fluoride
Hydrogen fluoride (HF), like water, is a polar molecule, and because its polarity can dissolve many ionic compounds. Its melting point is -84 Â ° C and its boiling point is 19.54 Â ° C (at atmospheric pressure); the difference between the two is slightly more than 100 K. HF also makes hydrogen bonds with its neighboring molecules, just like water and ammonia. It has been regarded as a possible solvent for life by scientists such as Peter Sneath and Carl Sagan.
HF is harmful to molecular systems living on Earth made of, but certain other organic compounds, such as paraffin wax, are stable with it. Like water and ammonia, liquid hydrogen fluoride supports acid-base chemistry. Using the definition of solvent and alkaline solvent systems, the function of nitric acid as a base when added to liquid HF.
However, hydrogen fluoride is rare, unlike water, ammonia, and methane.
Hydrogen sulfide
Hydrogen sulphide is the closest chemical analogue to water, but is less polar and a weaker inorganic solvent. Hydrogen sulfide is pretty much in Jupiter Io moons, and may be in the form of liquid short distances beneath the surface; and astrobiologist Dirk Schulze-Makuch suggested it as a possible solvent for life there. On a planet with a sea of ​​hydrogen-sulphide, the source of hydrogen sulfide can come from volcanoes, which in this case can be mixed with little hydrogen fluoride, which can help dissolve the minerals. The life of hydrogen sulfide may use a mixture of carbon monoxide and carbon dioxide as their carbon source. They may produce and live from sulfur monoxide, which is analogous to oxygen (O 2 ). Hydrogen sulfide, such as hydrogen cyanide and ammonia, suffers from the small temperature range in which it is liquid, however, such as hydrogen cyanide and ammonia, increases with increasing pressure.
Silicon dioxide and silicate
Silicon dioxide, also known as glass, silica, or quartz, is very abundant in the universe and has a large temperature range in which it is liquid. However, the melting point is 1,600 to 1,725 ​​° C (2,912 to 3,137 ° F), so it is not possible to make organic compounds at that temperature, because everything will decompose. Silicates are similar to silicon dioxide and some can have lower boiling points than silica. Gerald Feinberg and Robert Shapiro have suggested that liquid silicate rocks can serve as a liquid medium for organisms with chemicals based on silicon, oxygen, and other elements such as aluminum.
Other solvents or kosolvents
Other solvents are sometimes proposed:
- Supercritical fluids: supercritical carbon dioxide and supercritical hydrogen
- Simple hydrogen compounds: hydrogen chloride
- More complex compounds: sulfuric acid, formamide, methanol
- Very-very-temperature liquid: liquid nitrogen, and hydrogen
- High-temperature liquid: sodium chloride
Sulfuric acid in liquid form is very polar. It remains liquid at higher temperatures than water, its liquid range is 10 ° C to 337 ° C at 1 atm pressure, although above 300 ° C will slowly decompose. Sulfuric acid is known to be abundant in Venus clouds, in the form of aerosol droplets. In biochemistry using sulfuric acid as a solvent, an alkene group (C = C), with two carbon atoms joining a double bond, may function analogously with the carbonyl group (C = O) in a water-based biochemistry.
A proposal has been made that life on Mars may exist and use a mixture of water and hydrogen peroxide as its solvent. A 61.2% (by weight) water and hydrogen peroxide mixture has a freezing point of -56.5 Â ° C, and also tends to be super-cool rather than crystallized. It is also hygroscopic, an advantage in a rare water environment.
Supercritical carbon dioxide has been proposed as a candidate for alternative biochemistry because of its ability to dissolve organic compounds selectively and assist the function of enzymes and because of "super-Earth" or "super-Venus" planets with dense high-pressure atmospheres. may be common.
Other specs
Non-green photosynthesis
Physicists have noted that, although photosynthesis on Earth generally involves greenery, other colorful plants can also support photosynthesis, important for most life on Earth, and that other colors may be preferred in places that receive a mixture of different star radiation. from Earth. These studies show that, although blue photosynthesis plants will tend to be less, yellow or red plants are plausible.
Environment variable
Many Earth plants and animals undergo major biochemical changes during their life cycle in response to changes in environmental conditions, for example, by having a spore or hibernation state that can last for years or even thousands of years between the more active life stages. Thus, it is biochemically possible to sustain life in an environment that is only periodically consistent with life as we know it.
For example, frogs in cold climates can last for a long time with most of their body water in a frozen state, whereas the desert frogs in Australia can become dehydrated and dehydrated in dry periods, losing up to 75% of their fluids, but returning to life quickly rehydration in the wet period. Either type of frog will appear biochemically inactive (ie not alive) during the dormant period to anyone who has no sensitive means to detect a low metabolic rate.
Nonplanetary life
Dust and plasma-based
In 2007, Vadim N. Tsytovich and his colleagues propose that living human behavior can be exhibited by suspended dust particles in plasma, under conditions that may exist in space. Computer models show that, when the dust becomes charged, the particles can self-organize into microscopic helical structures, and the authors offer "rough sketches of possible models of reproduction of helical grain structures".
Scientists who have published this topic
Source of the article : Wikipedia